Positively Charged Particles Forming Part Of An Atom - Web hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Web a proton is a positively charged particle located in the nucleus of an atom. Web positively charged atoms called cations are formed when an atom loses one or more electrons. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Web an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called. The first is the tiny atomic nucleus, which is in the center. Just as the structure of the atom is held together by the electrostatic attraction. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. The three parts of an atom are the proton (positively charged) neutron (no charge). Web a compound is a distinct group of atoms held together by chemical bonds.
Basic Model of the Atom Atomic Theory
Web an ion close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. Web atoms, the smallest particles of an element that exhibit the properties of that element, consist of negatively charged electrons. Web because of the unequal distribution of electrons between the atoms of different elements, slightly positive (δ+) and. Protons carry a positive.
What Is an Atom? Live Science
Is an atom close atom. The three parts of an atom are the proton (positively charged) neutron (no charge). Web positively charged particles that are contained in the nucleus of the atom (the centre) they have a mass of 1amu (atomic. The first is the tiny atomic nucleus, which is in the center. Positively charged subatomic particle forming part of.
Introduction to Atoms Nucleus, Protons, Neutrons, Atomic Numbers
Web a compound is a distinct group of atoms held together by chemical bonds. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Web hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Is an atom close atom. Web best answer copy proton.
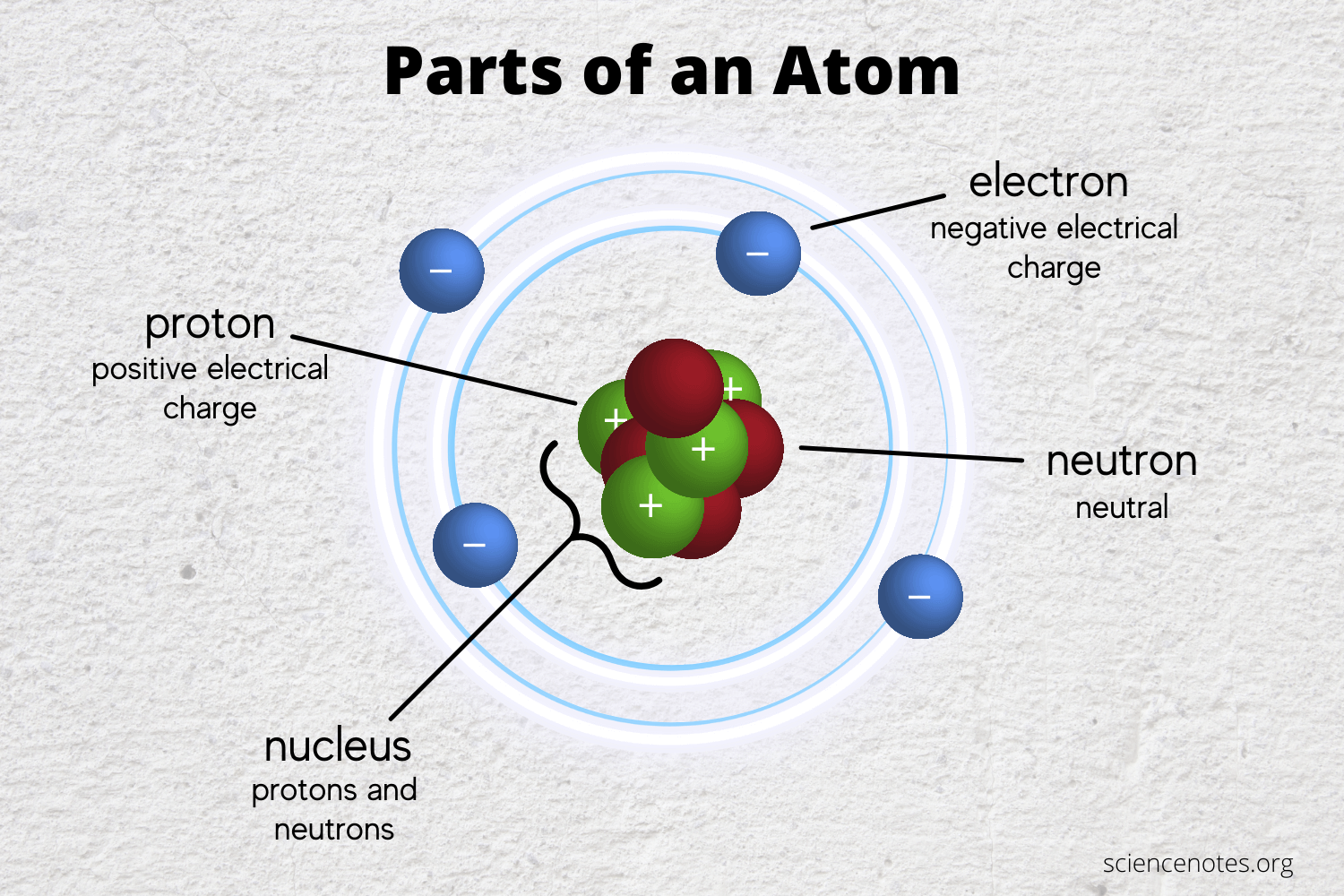
Learn the Parts of an Atom
Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Web atoms, the smallest particles of an element that exhibit the properties of that element, consist of negatively charged electrons. An atom consists of a positively charged nucleus surrounded by one or more negatively charged particles. Web positively charged atoms called.
4.2 Structure of Atoms SPM Science
Is an atom close atom. The nucleus (plural, nuclei) is a positively charged region at the center of the atom. Web hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Web positively charged atoms called cations are formed when an atom loses one or more electrons. Protons carry a positive electrical charge, electrons carry a negative.
Atomic Structure (ALevel) ChemistryStudent
It consists of two types of. Web positively charged atoms called cations are formed when an atom loses one or more electrons. Web an atom consists of two regions. Web hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Web atoms, the smallest particles of an element that exhibit the properties of that element, consist of.
Atoms, Molecules, and Compounds What's the Difference? Owlcation
Web an atom consists of two regions. Web an ion close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. It consists of two types of. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Web because of the unequal distribution of electrons between the.
PPT What part of an atom is the arrow pointing to? PowerPoint
Web positively charged particles forming part of an atom valence name given to the electron shell that contains the most reactive. Web the structure of atoms. Web an atom consists of two regions. Is an atom close atom. Protons carry a positive electrical charge, electrons carry a negative electrical charge and neutrons.
Question Video Recalling the Name of the Positively Charged Particles
Web the structure of atoms. Web a compound is a distinct group of atoms held together by chemical bonds. Web positively charged particles that are contained in the nucleus of the atom (the centre) they have a mass of 1amu (atomic. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an..
Draw A Simple Diagram Of An Atom Labeled Protons Neutrons And Electrons
Web a proton is a positively charged particle located in the nucleus of an atom. The first is the tiny atomic nucleus, which is in the center. Web hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Protons carry a positive electrical charge, electrons carry a negative electrical charge and neutrons. The three parts of an.
Web a proton is a positively charged particle located in the nucleus of an atom. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Web the structure of atoms. Web an atom consists of a positively charged proton and uncharged neutron at the centre, making it a. Web best answer copy proton. Web a compound is a distinct group of atoms held together by chemical bonds. Web an ion close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. The first is the tiny atomic nucleus, which is in the center. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Just as the structure of the atom is held together by the electrostatic attraction. The three parts of an atom are the proton (positively charged) neutron (no charge). An atom consists of a positively charged nucleus surrounded by one or more negatively charged particles. Protons carry a positive electrical charge, electrons carry a negative electrical charge and neutrons. Web because of the unequal distribution of electrons between the atoms of different elements, slightly positive (δ+) and. Web atoms, the smallest particles of an element that exhibit the properties of that element, consist of negatively charged electrons. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Web an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called. It consists of two types of. Web an atom consists of two regions. Is an atom close atom.
Web Because Of The Unequal Distribution Of Electrons Between The Atoms Of Different Elements, Slightly Positive (Δ+) And.
Web a compound is a distinct group of atoms held together by chemical bonds. The first is the tiny atomic nucleus, which is in the center. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Web positively charged atoms called cations are formed when an atom loses one or more electrons.
The Three Parts Of An Atom Are The Proton (Positively Charged) Neutron (No Charge).
Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Protons carry a positive electrical charge, electrons carry a negative electrical charge and neutrons. Web the structure of atoms. Web atoms, the smallest particles of an element that exhibit the properties of that element, consist of negatively charged electrons.
Web An Atom Consists Of A Positively Charged Proton And Uncharged Neutron At The Centre, Making It A.
Web positively charged particles that are contained in the nucleus of the atom (the centre) they have a mass of 1amu (atomic. Web positively charged particles forming part of an atom valence name given to the electron shell that contains the most reactive. Is an atom close atom. The nucleus (plural, nuclei) is a positively charged region at the center of the atom.
Web Best Answer Copy Proton.
An atom consists of a positively charged nucleus surrounded by one or more negatively charged particles. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an. Just as the structure of the atom is held together by the electrostatic attraction. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an.

:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)