Which Elements Can Form Acidic Compounds - Web acidic and basic property. Option b, rubidium can form basic compounds explanation: Web in particular, there are 7 elements that are called diatomic, meaning that on their own they will almost always form molecules consisting of two atoms. Basic compounds are those which have. They generally form acidic or neutral oxides with oxygen that that dissolve. Web hydrogen iodide or hydroiodic acid. Web common strong acids include hydrochloric acid, sulfuric acid, phosphoric acid, and nitric acid. Answer 3 people found it helpful profile brainly user report flag. Sulfur, arsenic, selenium, antimony, silicon. Elements w.this electron configuration have large +.
Group 1a 2a 3a Periodic Table Periodic Table Timeline
The chemical formula for acetic acid, a common acid found in vinegar, is c 2 h 4 o 2. Web sulfur (s), arsenic (as), selenium (se), and antimony (sb) are the elements that can form acidic compounds. Answer 3 people found it helpful profile brainly user report flag. Web most of the covalent hydrogen compounds are weak acids— in some.
CHEM1902 Acids, Bases and Solvent Systems
Check all that apply.* boron. Option b, rubidium can form basic compounds explanation: Web sulfur, arsenic, selenium, antimony answered by bot gpt 3.5 answer this question still need help? Web which elements can form acidic compounds? Web the elements that can form acidic compounds are;
Chemical Nomenclature and Chemical Formulas Owlcation
Basic compounds are those which have. Web which elements can form acidic compounds? How many oxygen atoms are there in three. Check all that apply.* boron. Elements w.this electron configuration have large +.
Acids — Definition & Overview Expii
Web oxides are binary compounds of oxygen with another element, e.g., co 2, so 2, cao, co, zno, bao 2, h 2 o, etc. Web the following elements that can form acidic compounds are the following: This is the chemical structure for hydrogen iodide, hi, which dissociates in. The chemical formula for acetic acid, a common acid found in vinegar,.
PPT Common Acids PowerPoint Presentation, free download ID4697679
Correct option is a) down the group, the metallic property increases due to the general increase in. When an element combines with oxygen forming a compound known as oxide ,. Web sulfur (s), arsenic (as), selenium (se), and antimony (sb) are the elements that can form acidic compounds. Basic compounds are those which have. Web oxides are binary compounds of.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
Elements w.this electron configuration have large +. They generally form acidic or neutral oxides with oxygen that that dissolve. Web metalloids flashcards | quizlet metalloids 5.0 (1 review) which property do metalloids share with nonmetals? Web which elements can form acidic compounds? Answer 3 people found it helpful profile brainly user report flag.
Naming Acid Compounds in Chemistry. YouTube
Web both can react to form acidic compounds. Web sulfur (s), arsenic (as), selenium (se), and antimony (sb) are the elements that can form acidic compounds. This is the chemical structure for hydrogen iodide, hi, which dissociates in. Recall that oxides of metals are basic and oxides or nonmetals are acidic;. Correct option is a) down the group, the metallic.
10 Common Acids and Chemical Structures
How many oxygen atoms are there in three. Check all that apply.* boron. This is the chemical structure for hydrogen iodide, hi, which dissociates in. Web acidic and basic property. Web the following elements that can form acidic compounds are the following:
PPT Unit 3 PowerPoint Presentation, free download ID5556477
They generally form acidic or neutral oxides with oxygen that that dissolve. These elements are in between. Web hydrogen iodide or hydroiodic acid. Web both can react to form acidic compounds. Elements w.this electron configuration have large +.
Solubility and pH
Web both can react to form acidic compounds. Check all that apply.* boron. Web the following elements that can form acidic compounds are the following: Web metalloids flashcards | quizlet metalloids 5.0 (1 review) which property do metalloids share with nonmetals? Web most of the covalent hydrogen compounds are weak acids— in some cases, such as methane, ch 4, so.
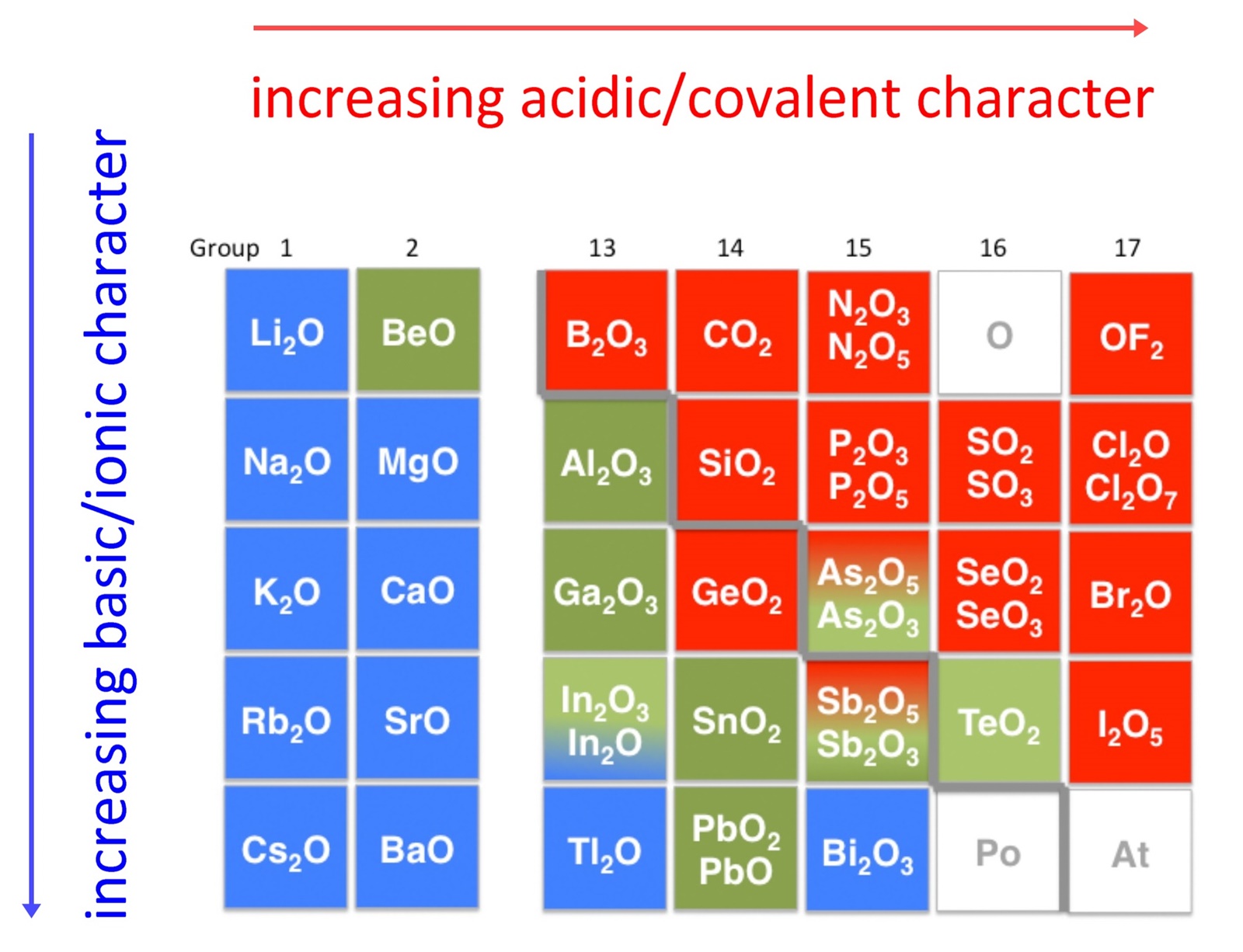
Option b, rubidium can form basic compounds explanation: Web which elements can form acidic compounds? Web compounds composed entirely of nonmetals are molecular substances (not ionic). How many oxygen atoms are there in three. Web sulfur (s), arsenic (as), selenium (se), and antimony (sb) are the elements that can form acidic compounds. Web hydrogen iodide or hydroiodic acid. When an element combines with oxygen forming a compound known as oxide ,. This is the chemical structure for hydrogen iodide, hi, which dissociates in. Correct option is a) down the group, the metallic property increases due to the general increase in. Recall that oxides of metals are basic and oxides or nonmetals are acidic;. Web metalloids flashcards | quizlet metalloids 5.0 (1 review) which property do metalloids share with nonmetals? Web these elements vary in their reactions with oxygen. They generally form acidic or neutral oxides with oxygen that that dissolve. Web in particular, there are 7 elements that are called diatomic, meaning that on their own they will almost always form molecules consisting of two atoms. Web sulfur, arsenic, selenium, antimony answered by bot gpt 3.5 answer this question still need help? Basic compounds are those which have. Web the elements that can form acidic compounds are; The table shows columns that brenda uses for her notes on the properties of. Web most of the covalent hydrogen compounds are weak acids— in some cases, such as methane, ch 4, so weak that their acidic. Web the following elements that can form acidic compounds are the following:
Answer 3 People Found It Helpful Profile Brainly User Report Flag.
Sulfur, arsenic, selenium, antimony, silicon. These elements are in between. Web the following elements that can form acidic compounds are the following: Web the elements that can form acidic compounds are;
When An Element Combines With Oxygen Forming A Compound Known As Oxide ,.
Web metalloids flashcards | quizlet metalloids 5.0 (1 review) which property do metalloids share with nonmetals? An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of. Check all that apply.* boron. Web study with quizlet and memorize flashcards containing terms like *which elements are metalloids?
They Generally Form Acidic Or Neutral Oxides With Oxygen That That Dissolve.
Web compounds composed entirely of nonmetals are molecular substances (not ionic). Web hydrogen iodide or hydroiodic acid. How many oxygen atoms are there in three. Web sulfur (s), arsenic (as), selenium (se), and antimony (sb) are the elements that can form acidic compounds.
You Can Ask A New Question Or.
The table shows columns that brenda uses for her notes on the properties of. Web which elements can form acidic compounds? Elements w.this electron configuration have large +. Recall that oxides of metals are basic and oxides or nonmetals are acidic;.







/common-acids-and-chemical-structures-603645_FINAL-54e6b0b3351b49dbb6cef54fd4817404.png)

